[ Show ]
Support VoyForums
[ Shrink ]
VoyForums Announcement:
Programming and providing support for this service has been a labor
of love since 1997. We are one of the few services online who values our users'
privacy, and have never sold your information. We have even fought hard to defend your
privacy in legal cases; however, we've done it with almost no financial support -- paying out of pocket
to continue providing the service. Due to the issues imposed on us by advertisers, we
also stopped hosting most ads on the forums many years ago. We hope you appreciate our efforts.
Show your support by donating any amount. (Note: We are still technically a for-profit company, so your
contribution is not tax-deductible.)
PayPal Acct:  Feedback:
Feedback: 
Donate to VoyForums (PayPal):
[ Next Thread |
Previous Thread |
Next Message |
Previous Message ]
Date Posted: 04:24:37 07/16/06 Sun
Author: Nitrous Oxide
Subject: Modern Mechanix
Nitrous Oxide - 

Ewan Cameron and Paul
May
School of Chemistry,
University of Bristol

HTML-only
version Chime
version Chemsymphony
(java) version

"I am sure the air in heaven must be this wonder working gas of
delight". So wrote poet Robert Southey of nitrous oxide, N2O,
also known as nitrogen oxide, dinitrogen monoxide, hyponitrous acid anhydride
and facticious air. However, its most well known name is 'laughing gas' due to
its intoxicating effects when inhaled.
Laughing Gas

Nitrous oxide, N2O, is a colorless, almost
odorless gas, that was first discovered in 1793 by the English scientist and
clergyman Joseph Priestley (who was also famous for being the first to isolate
other important gases such as oxygen, carbon monoxide, carbon dioxide, ammonia,
and sulfur dioxide). Priestley made N2O by heating ammonium nitrate
in the presence of iron filings, and then passing the gas that came off (NO)
through water to remove toxic by-products. The reaction he observed was:
2NO + H2O + Fe 
N2O + Fe(OH)2
After initial trials, Priestley thought that N2O could be used as
a preserving agent, but this proved unsuccessful.
 Following
Following
Priestley's discovery, Humphry Davy of the Pneumatic Institute in Bristol,
England, experimented with the physiological properties of the gas, such as its
effects upon respiration. He even administered the gas to visitors to the
institute, and after watching the amusing effects on people who inhaled it,
coined the term 'laughing gas'! Davy even noted the anaesthetic effects of the
gas: "As nitrous oxide in its extensive operation appears capable of
destroying physical pain, it may probably be used with advantage during surgical
operations in which no great effusion of blood takes place".
 However,
However,
despite this observation, for the next 40 years or so the primary use of N2O
was for recreational enjoyment and public shows. So called nitrous oxide
capers took place in travelling medicine shows and carnivals, where the
public would pay a small price to inhale a minute's worth of the gas. People
would laugh and act silly until the effect of the drug came to its abrupt end,
when they would stand about in confusion. Many famous people (of their time) and
dignitaries from Clifton and Bristol came to inhale Davy's purified nitrous
oxide for recreational purposes.

Nitrous Oxide as an Anaesthetic
Nitrous oxide found a more scientific use as an anesthetic in clinical
dentistry and medicine in the early 1840s. The story goes that at that time, a
medical school dropout called Gardner Quincy Colton went around the country
putting on nitrous oxide exhibitions. In 1844 he happened to put on a
demonstration in Hartford, Connecticut, and in the audience that day was a local
dentist named Horace Wells. Dr. Wells watched with interest as one of the
volunteers, a man named Samuel Cooley, inhaled the gas, and, while still under
the effects of the N2O, injured his leg when he staggered into some
nearby benches. When he went back to his seat next to Dr. Wells, Cooley appeared
to be unaware of the injury until the effects of the gas wore off. Dr. Wells
immediately realised that N2O might possess painkilling qualities,
and so after the demonstration, Wells approached Colton and invited him to
participate in an experiment the next day. Colton agreed, administered nitrous
oxide to Dr. Wells while another local dentist extracted one of Wells' molars.
Dr. Wells experienced no pain during the procedure, and the birth of N2O
as a dental and medical painkiller had arrived.
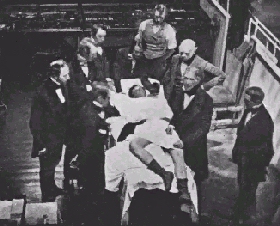
| Early surgical operations, such as this one from
1850, used ether as an anesthetic. This was dangerous, because ether is
highly explosive. So a new anesthetic was needed that was not so
dangerous to use. |
The story does not end happily, however. In January 1845, Dr. Wells
demonstrated his discovery of the effects of nitrous oxide at the Harvard
Medical School in Boston. A patient was anesthetised and a tooth was extracted,
but during the demonstration the patient complained that he felt some
discomfort. Even though the experiment had been successful (in that the patient
had only felt slight discomfort and not excruciating pain), the
suspicious audience were unhappy, and booed Wells from the stage. This public
humiliation eventually led to Dr. Wells losing his reputation as a profession
dentist, and finally to his suicide three years later. Ironically, 150 years
after his premature death, his discovery would be adopted by dental practices
worldwide, and Wells would be given the accolade - the "Discoverer of
Anesthesia".
Nitrous oxide is a very safe and popular agent still utilized by dentists
today. It is much less toxic than alternatives, such as chloroform, with far
less risk of explosion than ether. The main use for N2O is usually as
a mild sedative and analgesic. It helps to allay anxiety that many patients may
have toward dental treatment, and it offers some degree of painkilling ability.

A Boost for Fast Cars 
At room temperature, N2O is quite unreactive with
most substances, including alkali metals, halogens, and even ozone. It is
therefore widely used as a propellant in aerosol cans in place of the CFCs which
can damage the ozone layer. When heated sufficiently, however, N2O
decomposes exothermically to N2 and O2.
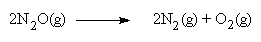
 If
If
this reaction occurs in the combustion chamber of an automobile, 3 moles of gas
would be produced from 2 moles, providing an extra boost to the piston, as well
as liberating more heat. It also has a number of other benefits. The increased
oxygen provides more efficient combustion of fuel, the nitrogen buffers the
increased cylinder pressure controlling the combustion, and the latent heat of
vaporisation of the N2O reduces the intake temperature. Therefore N2O
is occasionally injected into the fuel
lines of racing cars to give more power to the engine and to give the car
exceptional acceleration. Some
details on how this works can be found here.
References:
- I.E. Eger, Nitrous Oxide (New York, Edward Arnold Ltd, 1985).

This is a Popular Science article from 1949 which teaches budding young
chemists how to make nitrous oxide. It even helpfully explains that the gas
produces “a feeling of exhilaration when inhaled”.
Other articles in this series include:
- The crystal which eliminates the need for sleep.
- The dust that lets you lift a car.
- The weed that makes you feed.
- The liquid that gives you control of time and space
[
Next Thread |
Previous Thread |
Next Message |
Previous Message
]
Replies:
 Feedback:
Feedback: 
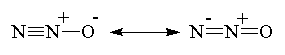


 If
If